
Research
De-coding nature’s unbelievable capabilities to produce nanoscale machines
Let's go down the rabbit hole...

Measuring Intrinsically Disordered Proteins Interactions
One emerging class of biomolecules whose function relies on weak interactions involves intrinsically disordered proteins. These proteins display an amino acid sequence that does not lead to singular, stable 3D structures. Moreover, their properties and function are still largely unknown as they violate the conventional sequence-structure-function paradigm. Structural flexibility and plasticity, and the lack of a secondary ordered structure, suggest a major functional advantage for these proteins, enabling them to interact with a broad range of binding partners. We study new means to characterize intrinsically disordered protein interactions aiming to understand the relation between their sequence and their biological function

Calculating Entropy from Computer Simulation of Physical Systems
Entropy is a basic quantity in physics. Nonetheless, calculating it from computer simulation is a demanding, and sometimes impractical, task. We use information theories and algorithms to design new means to calculate entropy efficiently and without the need for additional simulation. These new calculations can enable us to estimate free energy in complex systems, such as protein folding simulations, as means to identify the energy landscape of the problem, the native protein structures and its alterations in diseased state.

Intrinsically Disordered Polypeptide Amphiphiles
Polypeptide amphiphiles are a growing class of molecules that can assemble into biologically functional structure. The molecules' "headgroup", usually a bio-inspired peptide, engineered to fulfill one or more active roles, is chemically conjugated to a hydrophobic "tail". The tails are usually single chain fatty acids or double chain like those found in lipids. Due to their highly flexible design scheme, Polypeptide amphiphiles self-assembly can be tailored for novel biomedical research. Inspired from nature and our recent studies, we conjugate disordered proteins domains to hydrocarbon to design Intrinsically Disordered Polypeptide amphiphiles. The multidimensional functionality of these molecule is investigated mainly using SAXS, TEM and DLS under various environmental conditions.

Supramolecular Forces within Intermediate Filaments
Cytoskeletal proteins are the major structural components of cells. In recent years, much fruitful research has dealt with the structures and interactions of microtubules (25 nm in diameter hollow nanotube) and actin filaments (8 nm in diameter). However, little is known about the third component of the cytoskeleton, intermediate filaments, which, in contrast to actin and microtubules, are cell specific. Intermediate filaments principal function is to mechanically support the cell and its membrane. The common denominator to all intermediate filaments is that they form a 10 nm filament core while the difference is found mainly in the unstructured brushes. We aim to understand the forces and interactions responsible for the assemblies of the different intermediate filaments from the single filament properties to the condensed hydrogel network composed from many interacting filaments.

Biomolecules Supramolecular Assemblies
Biomolecules Supramolecular Assemblies
In many significant biological functions the four basic building blocks (proteins, lipids, sugars and nucleic acids) aggregate to form supramolecular structures and assemblies. The forces and interactions responsible for these assemblies are composed of a set of interactions with energy scales from the order of thermal fluctuation (few KBT) to specific covalent bonds on the order of 100’s KBT. Moreover, relevant length scales in biological systems span over many orders of magnitude, from the single amino acid through polypeptide chains, protein complexes, organelles and up cells and organs. These different length scales present an enormous challenge both experimentally and theoretically. Therefore, in order to properly study biological systems and the interactions within them, it is important to have complementary techniques covering different length-scales and energies, with proximity to their natural environment. In our laboratory we purify the subunit building-blocks using various biochemical and molecular procedures and reassemble them in various conditions to study their supramolecular forces, dynamics and steady-state structures as appear in healthy and diseased states.

Super Resolution Small Angle X-ray Scattering

X-ray scattering and diffraction have been a leading tool in the past century in characterizing material structures. In our laboratory, we use a small-angle x-ray scattering (SAXS) technique to cover length scales from 0.1-100 nm. This technique is suitable to measure weak scattering from biological systems in their natural environment. Detailed analysis and advanced computational techniques are regularly used to convert the reciprocal space to real-space structures and enable studies on the nature of the interactions within the biological assemblies. The current project aims to improve the SAXS angular resolution by using a novel image reconstruction algorithm that is coupled to hardware design.
On the Relation between Multiple Sclerosis and Membrane Physics
Multiple sclerosis (MS) is an autoimmune neurodegenerative disease which is unpredictable and incurable that disrupts the electrical signaling between the brain and the body. While the cause to MS is still unknown, we been studying in the last years innovative perspective on how the disease initiate that can help develop novel early diagnostic and possible treatment to the disease. Our studies aim to resolved what are the minimal components needed to emulate the dysfunction of the myelin sheaths as they appear, for example, in MS. As with any biological system, the myelin sheaths that enwrap the long axon in nerve cells, are a complicated system with many different components. From clinical studies, it is known that several components are altering during a diseased state. Unfortunately, what was not known is which of the component is redundant for the myelin sheaths function and what alterations cause devastating results. Using X-ray scattering and electron microscopy, we study the delicate balance of intermolecular forces that drive myelin structure from its lamellar order to inverted hexagonal phase in diseased state.

Metastability of supercooled membranes
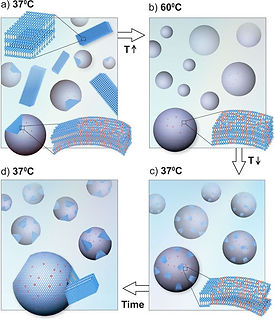
Metastable states in first-order phase-transitions have been traditionally described by classical nucleation theory. We study the controllable behavior of the long-lived metastable liquid-crystalline phase of phospholipids when arranged in multi-lamellar vesicles, and the ensuing cooperative transition to the crystalline state. This delayed crystallization of phospholipids upon super-cooling is an interesting case, since the extended timescales allow access into the dynamics. Experimentally, we find that the delay in crystallization is a bulk phenomenon, which is tunable and can be manipulated to span two orders of magnitude in time by changing the quenching temperature, solution salinity, or adding a secondary phospholipid. Our results reveal the robust persistence of the metastability, and showcase the apparent deviation from CNT. Since phospholipids are used as a platform for drug-delivery, a programmable design of cargo hold and release can be of great benefit.

